4 Steps on How Copper Plating Solution Works
- Plating |
- Aug 9, 2021

Copper plating or copper electroplating is a massive industry that is rising enormously across the globe. It is considered a highly refined and effective method in the metal coating industry. However, copper in itself is very expensive because of the limited supply and high demand. The reason the demand for copper is high is that it is a vital component used for everything from plumbing, wiring, electronics to industrial fertilizers and pesticides. Copper is considered a miracle metal because of its unique properties this metal is malleable, it conducts electricity, resists corrosion and the best part is it can easily take on a variety of shapes. So, copper plating industry is on rising. Basically, copper plating is a process in which a thin layer of copper is plated or placed on the surface of a metal or a non-metal. This article will explain what is copper plating? and how does copper plating solution work?
Copper electroplating:
Copper electroplating is a common way to finish anything by using electricity to place a thin layer of copper on a surface that conducts electricity. Copper is often used in electronics, vehicles, and decorative goods because metal is robust and conducts electricity well.
Copper electroplating is done through the use of electricity or by passing an electric current in a solution called an electrolyte. In copper electroplating solution, a container of water with the copper rod and the item that needs copper plating is placed. This water contains an ionic solution that helps the electricity to flow directly from the copper rod to the plating item. By dipping two electrodes into the electrolyte and connecting it to a circuit with electricity or a battery, it dissolves the copper rod and physically transports the copper ions to the item that needs the plating. Hence, a thin, solid, metallic copper film or coating is formed on the surface of the item. In short, the concept of copper electroplating solution is – when an item is submerged into an electrolyte bath, where electricity is directly applied to make the copper ions migrate and deposit on the surface of the metal that needs copper plating.
Also Read- Metal Electroplating: An Expert Guide to the Process
This process of copper electroplating is very interesting and it is also so astonishing to see how a mundane piece of metal can be transformed into an expensive product. Now we will have a closer look at how the copper plating solution works.
EXPLORE OUR PLATING SERVICES
How Copper Plating Solution Works:
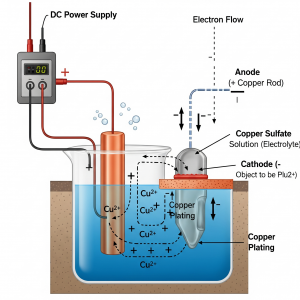
STEP 1: Choosing the right electrolyte and the right electrodes is the first step towards electroplating. So, for copper plating, an electrolyte that is made from a solution of copper salt is necessary.
STEP 2: The next step is to check that the electrode is completely clean and this can be done by dipping the electrode into an alkaline solution or a strong acid to make it dirt-free. This is an important step because if the electrode is not clean, then the copper atoms will not form a good bond on it and may erode or rub off. Hence, when the electrode is clean it will help the copper atoms to effectively bond on it and keep the copper plating strong and intact.
STEP 3: Once the electrode is clean, then comes the actual process of electroplating. For this we require 1) Two electrodes which are of different conducting materials, 2) An electrolyte or the solution containing the salt of copper, and 3) A battery or an electricity supply. For example, since here we are focusing on copper electroplating and if we want copper plating on a piece of iron, then we need a copper electrode, an iron electrode that is clean, and a solution of copper salt like copper sulfate solution. In this process the copper becomes the positive electrode or it is also called the anode and the iron becomes the negative electrode or as it is called the cathode.
DISCUSS YOUR PLATING NEEDS? CONTACT US!
STEP 4: In this final step, once the two types of electrodes are dipped in the solution then comes the next step of connecting it to electricity. So, as soon as the setup is ready, electricity is passed and the copper sulfate solution splits into ions and gets attracted to the iron electrode, which slowly deposits a thin layer of copper plating on it. Copper electroplating takes time and it mostly depends on the concentration of the electrolyte and the strength of the electricity.
Copper Electroplating Techniques
Fundamentally, electroplating relies on a basic electrolysis setup that combines the correct anode, cathode, and copper electrolyte. However, to meet different project specifications, a precise command of the plating rate and surface adhesion is often required.To achieve this, various additives and chemicals serve as levelers, accelerators, or suppressors to ensure the desired outcome.
Here are the four main techniques that industry experts use for copper plating metals.
Dual Damascene Plating
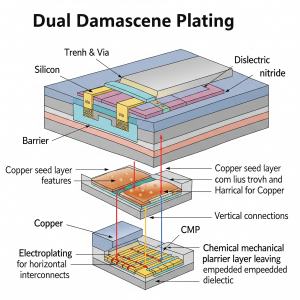
The dual damascene technique is used for applications where certain features of the part are important for electroplating. It’s typically applied to smaller components, often in the nanometer range, and in semiconductors that require distinct conductivity and resistance for key functions.
In this method, suppressors, accelerators, and levelers work in tandem to facilitate bottom-up plating. As shown in the illustration, suppressors on the sidewalls inhibit the copper deposition rate, while levelers at the top of the surface work to reduce the accumulation of copper ions.
Through-Silicon Via Plating
![]()
The silicon via plating technique is similar to the aforementioned dual damascene method but is slower and designed for larger features, measured in micrometers rather than nanometers.
As the illustration demonstrates, both suppressors and levelers collaborate to decrease the deposition rate on the sides. The accelerators enable the necessary bottom-up filling. The process is lengthy, requiring approximately an hour to complete, which is why the concentration of accelerators at the bottom is kept low.
Copper Pillar Plating
The copper pillar plating process is both time-consuming and complicated because of its requirements. In this case, you need to ensure the part’s coplanarity and provide high plating rates without sacrificing uniformity.
A successful plating process gives you the precise thickness of the layer on the surface, and you need a setup where the part continues to spin for this. This rotation, combined with the incoming flow, creates a uniform current density that consistently produces the required results.
Simple Plating Techniques
Different “recipes” for the liquid bath give you different results.
Acid Copper Plating: This is the most common and fastest way. Think of it as a strong, fast-acting liquid that quickly gives you a smooth, shiny copper layer. It’s used for most things that need a thick copper coat.
Alkaline Copper Plating: This method is a bit slower but makes the copper stick very, very well. It’s often used for a “primer” coat, a thin first layer that makes sure the next, thicker layer of copper doesn’t peel off.
Electroless Plating: This is a special method that doesn’t use electricity at all! It’s like a “magic liquid” that can coat objects that aren’t metal, like plastic. It’s used to put a thin layer of copper on things that can’t conduct electricity so that they can be “painted” with a thicker copper layer later on.
Also Read- Rack and Barrel Plating Services: Which One is Right For You?
Electroplating can be used for various purposes either for protecting metal or for decorative purposes and that is why it is important that the metal that gets electroplated must remain durable and visually bright. Hence, copper plating is used in automobiles, aircraft, telecommunication, consumer goods to make cheap metals look expensive.
FAQs
Q1: What chemicals are used in copper electroplating?
Copper plating chemicals that make the deposition even are often made with copper sulphate, sulfuric acid, and other compounds.
Q2. What are the typical applications of copper plating?
Electronics, semiconductors, vehicle parts, circuit boards, and cosmetic coatings all need copper electroplating.
Q3. On what factors does plating quality depend?
The quality of plating depends on the temperature, the composition of the bath, the preparation of the electrodes, and the chemicals used for copper plating.
Q4: What kinds of things do you use for electrodes?
When you electroplate copper, you normally need a copper anode and a workpiece that can conduct electricity (cathode).
Q5. Why is cleaning the electrode essential?
Cleaning well removes oils, oxides, and other dirt, which makes sure that the copper sticks well and is equally spread out.
Ujjwal handles crucial roles like AGM Marketing, researcher, and is an author for KDDL – Eigen. He currently works with Eigen for implementing proven techniques and strategies for marketing plans on online and offline platforms. An expert in efficiently executing SEO, SEM, email marketing, social media marketing, PR marketing, Print campaigns, etc. Ujjwal has coordinated an efficient marketing team on various creative campaigns and programmatic buying to support various digital cross-promotion efforts. Implement efficient search optimization strategies with the help of collateral material and metrics.
In his former years, Ujjwal has years of experience in a managerial role for several reputed companies. His years of experience combined with the flair of writing help him come up with result oriented strategies for Eigen.